ADVERSE REACTIONS
AKEEGA® DAT offers a manageable safety profile consistent with the known safety profile of each therapy1,2
Discontinuations and reductions in patients with BRCAm mCRPC1
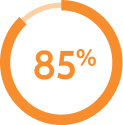 remained on AKEEGA® without discontinuing due to ARs*
remained on AKEEGA® without discontinuing due to ARs*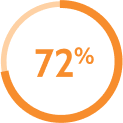 did not need a dose reduction with AKEEGA® due to ARs†
did not need a dose reduction with AKEEGA® due to ARs† remained on AKEEGA® without interruption due to ARs‡
remained on AKEEGA® without interruption due to ARs‡Adverse reactions (>10%) in patients with BRCAm mCRPC receiving AKEEGA®1
| AKEEGA® + P (n=113) | Placebo + AAP (n=112) | |||
|---|---|---|---|---|
| Adverse Reaction | All Grades % | Grade 3-4 % | All Grades % | Grade 3-4 % |
| Musculoskeletal paina | 44 | 4 | 42 | 5 |
| Fatiguea | 43 | 5 | 30 | 4 |
| Constipation | 34 | 1 | 20 | 0 |
| Hypertensiona | 33 | 14 | 27 | 17 |
| Nausea | 33 | 1 | 21 | 0 |
| Edemaa | 17 | 0 | 9 | 0 |
| Dyspneaa | 15 | 1 | 8 | 3 |
| Decreased appetite | 15 | 2 | 8 | 0 |
| Vomiting | 15 | 0 | 7 | 1 |
| Dizzinessa | 14 | 0 | 10 | 0 |
| COVID-19a | 13 | 7 | 9 | 4 |
| Abdominal paina | 12 | 2 | 12 | 1 |
| Hemorrhagea | 12 | 2 | 8 | 1 |
| Headache | 12 | 1 | 9 | 0 |
| Urinary tract infectiona | 12 | 3 | 9 | 1 |
| Cougha | 12 | 0 | 6 | 0 |
| Insomnia | 12 | 0 | 4 | 0 |
| Weight decreased | 10 | 1 | 4 | 1 |
| Arrhythmiaa | 10 | 2 | 4 | 1 |
| Fall | 10 | 1 | 13 | 4 |
| Pyrexiaa | 10 | 2 | 6 | 0 |
aIncludes multiple similar terms.
Most adverse events were mild or moderate (Grade 1 or 2) and manageable1
LAB ABNORMALITIES
Select lab abnormalities (>10%) that worsened from baseline in patients with BRCAm mCRPC receiving AKEEGA®1
| AKEEGA® + P (n=113)a | Placebo + AAP (n=112)a | |||
|---|---|---|---|---|
| Laboratory Abnormality | All Grades % | Grade 3-4 % | All Grades % | Grade 3-4 % |
| Hematology | ||||
| Hemoglobin decreased | 67 | 26 | 53 | 7 |
| Lymphocyte decreased | 55 | 22 | 32 | 13 |
| WBC decreased | 48 | 6 | 18 | 0.9 |
| Platelets decreased | 37 | 8 | 22 | 1.8 |
| Neutrophils decreased | 32 | 7 | 16 | 2.7 |
| Chemistry | ||||
| ALP increased | 34 | 1.8 | 29 | 1.8 |
| Creatinine increased | 30 | 0 | 13 | 1.8 |
| Potassium increased | 25 | 0.9 | 21 | 3.6 |
| Potassium decreased | 20 | 5 | 20 | 5 |
| AST increased | 20 | 1.8 | 25 | 2.7 |
| ALT increased | 18 | 0.9 | 17 | 4.5 |
| Bilirubin increased | 18 | 0 | 10 | 0.9 |
aThe denominator used to calculate the rate varied from 111 to 112 for placebo + AAP and 113 for AKEEGA® + prednisone based on the number of patients with a baseline value and at least one post-treatment value.1
*Adverse reactions which resulted in permanent discontinuation of AKEEGA® in >2% of patients included COVID-19 (4.4%), anemia (2.7%), asthenia (2.7%), and vomiting (2.7%).1
†One case of MDS/AML occurred in the placebo + AAP group. MDS/AML, including cases with fatal outcome, has been observed in patients treated with niraparib, a component of AKEEGA®. See Prescribing Information for niraparib for information on MDS/AML when used as a single agent in other indications.1,3
‡Adverse reactions which required dose reductions in >2% of patients included anemia (12%), thrombocytopenia (4.4%), and fatigue (2.7%).1
References:
- AKEEGA® [Prescribing Information]. Horsham, PA: Janssen Biotech, Inc.
- Chi KN, Rathkopf D, Smith MR, et al. Niraparib and abiraterone acetate for metastatic castration-resistant prostate cancer. J Clin Oncol. 2023;41(18):3339-3351. doi:10.1200/JCO.22.01649
- Chi KN, Sandhu S, Smith MR, et al. Niraparib plus abiraterone acetate with prednisone in patients with metastatic castration-resistant prostate cancer and homologous recombination repair gene alterations: second interim analysis of the randomized phase III MAGNITUDE trial. Ann Oncol. 2023;34(9):772-782. doi:10.1016/j.annonc.2023.06.009

