Progression-free survival
AKEEGA®* significantly reduced the risk of disease progression or death in patients with BRCAm mCRPC1


PRIMARY ANALYSIS†
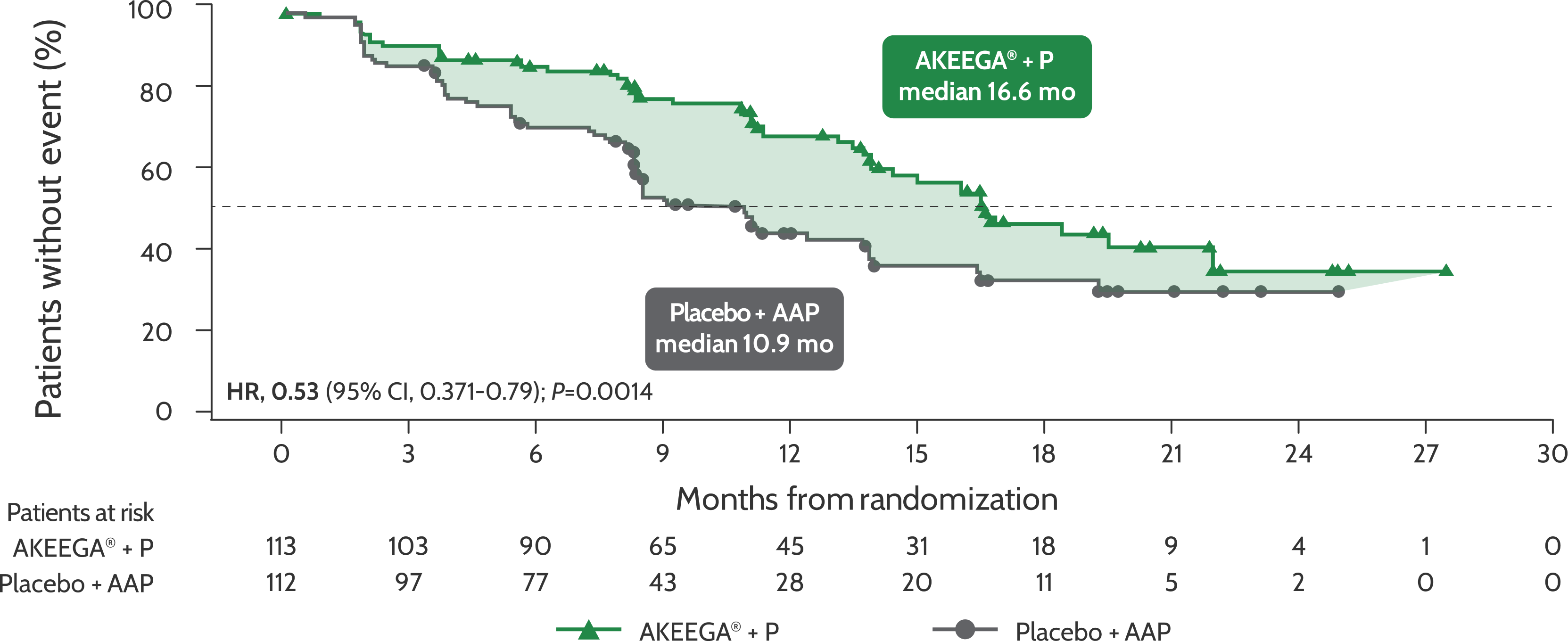
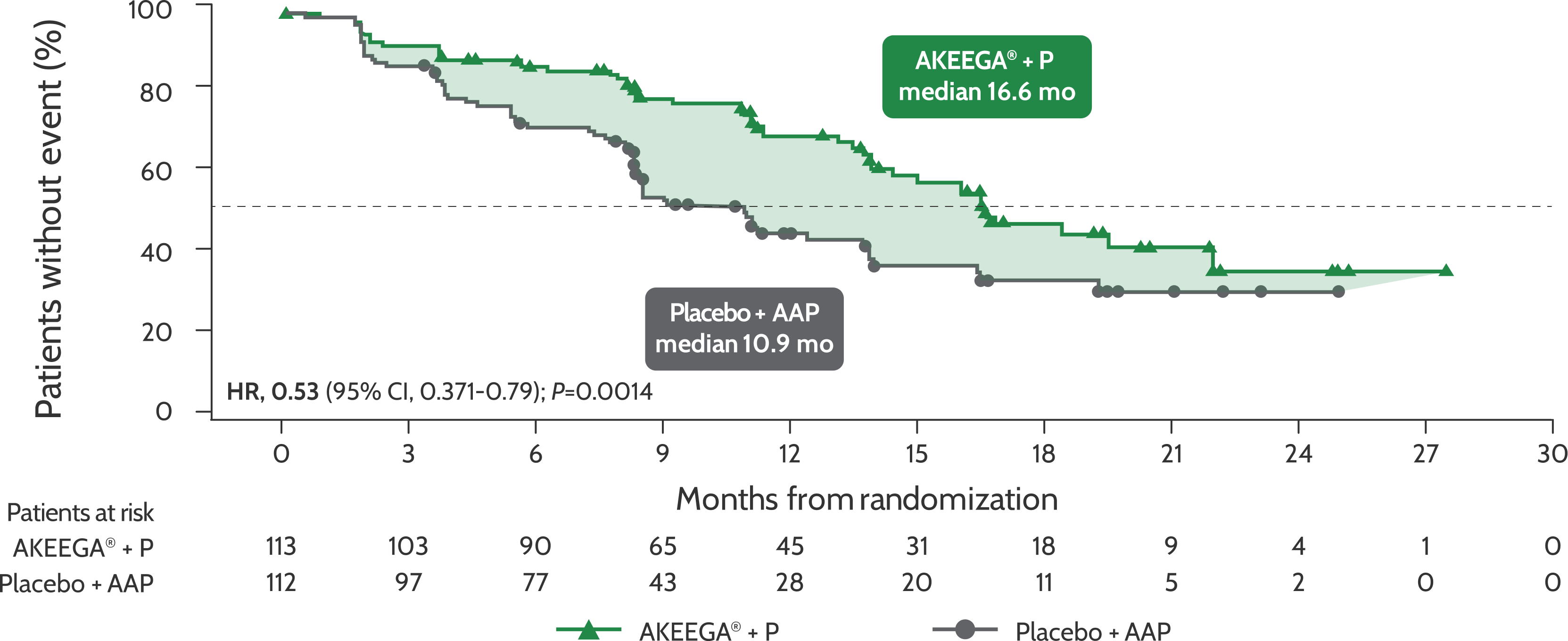
BICR-assessed rPFS in patients with BRCAm mCRPC1
Interim Analysis 2‡: Primary Endpoint
Risk of disease progression and/or death in the BRCAm mCRPC population: AKEEGA®* vs placebo + AAP2,3
rPFS in patients with BRCAm mCRPC


Statistical significance has not been established for the rPFS endpoint in the interim analysis 2 and this is not included in the full AKEEGA® Prescribing Information. This should be viewed in the context of patient management, overall physical condition, and clinical course of the patient.
Statistical significance has not been established for the rPFS endpoint in the interim analysis 2 and this is not included in the full AKEEGA® Prescribing Information. This should be viewed in the context of patient management, overall physical condition, and clinical course of the patient.
OVERALL SURVIVAL FINAL ANALYSIS§: SECONDARY ENDPOINT
Overall survival: AKEEGA®* vs placebo + AAP1
The median overall survival (OS) in the AKEEGA®* arm was 30.4 months (95% CI, 27.6-NE) and 28.6 months (95% CI, 23.8-33.0) in the placebo + AAP arm, with an OS HR of 0.79 (95% CI, 0.55-1.12).1
Statistical significance has not been established for this endpoint. This should be viewed in the context of patient management, overall physical condition, and clinical course of the patient.
Why Multivariate Analysis (MVA) Matters
MVA was used to adjust for key imbalances in baseline characteristics between arms including ECOG status, bone metastases, PSA, and visceral metastases. The prespecified multivariate analysis is based on modeling data and was not designed to assess safety or draw statistical significance. Due to the limitations, it is not possible to draw firm conclusions about the results of this analysis. These data do not appear in the AKEEGA® PI.4,5
Multivariate analysis produced an OS HR of 0.66 (95% CI, 0.46-0.95; P=0.024).4,5||¶
Other endpoints:
ADDITIONAL SECONDARY ENDPOINTS
Time to symptomatic progression in patients with BRCAm mCRPC4,5


Statistical significance has not been established for these endpoints and they are not included in the full AKEEGA® Prescribing Information. They should be viewed in the context of patient management, overall physical condition, and clinical course of the patient.
Time to initiation of chemotherapy in patients with BRCAm mCRPC4,5


More than 50% of patients continued therapy with AKEEGA® plus prednisone, hence median time to initiation of chemotherapy was not estimable.
Statistical significance has not been established for these endpoints and they are not included in the full AKEEGA® Prescribing Information. They should be viewed in the context of patient management, overall physical condition, and clinical course of the patient.
Study design
AKEEGA® 2-in-1 tablets offer improved benefit for patients with 1L BRCAm mCRPC vs placebo + AAP1
The MAGNITUDE trial was intentionally designed to study patients with BRCAm mCRPC, like the ones you may see in your practice1,6
MAGNITUDE enrolled the largest BRCAm population in PARPi combination studies to date2
53% (225/423) of patients enrolled in the HRR+ cohort had BRCA gene mutations1
MAGNITUDE Study Design3,6


Study start: February 2019; clinical data cutoff for Interim Analysis 1: October 8, 2021; Interim Analysis 2: June 17, 2022, Final Analysis: May 15, 2023.4,5
aPatients were prospectively tested by plasma, tissue, and/ or saliva/whole blood. Patients negative by plasma only were required to test by tissue to confirm HRR BM status. Tissue and plasma assays used included FoundationOne tissue test (FoundationOne®CDx), Resolution Bioscience liquid test (ctDNA), AmoyDx® blood and tissue assays, Invitae germline testing (blood/saliva), or local lab BM test results demonstrating a pathogenic germline or somatic alteration outlined in the study protocol.
bPatients in these cohorts received niraparib 200 mg once daily with AA 1,000 mg once daily plus prednisone 5 mg twice daily.
Key elements of MAGNITUDE1,2,6
Prior chemotherapy and novel hormonal agents, including ARis, allowed in the mCSPC or nmCRPC settings
Prospective genetic testing stratified patients based on HRR mutations
Up to 4 months of AAP permitted in first-line mCRPC to allow time for genetic testing, if needed
Designed to determine which group of patients would benefit from a PARPi + AAP
Patients enrolled in the MAGNITUDE trial were prospectively tested for HRR status1,6
- Patients were tested using FoundationOne®CDx tissue assay or other clinical trial assays
- Enrollment in the non–HRR-altered cohort was stopped when predetermined criteria of the futility analysis were met
Baseline characteristics
Select baseline characteristics in BRCA1/2 subgroup2
AKEEGA® + P (n=113)
Placebo + AAP (n=112)
ECOG PS 1
39%
29%
Bone metastases
88%
83%
Visceral metastases
23%
20%
Median PSA (µg/L)
18.7
14.1
Prior AAP for 1L mCRPCa
27%
26%
aPatients could have received up to 4 months of AAP in the mCRPC setting before study entry.
*AKEEGA® is indicated with 10 mg prednisone daily.1
†The median duration of follow-up in the BRCA1/2 subgroup at the primary analysis was 16.7 months.2
‡The median follow-up in the BRCA1/2 subgroup at interim analysis 2 was 24.8 months (range 0.5-36.8 months).2
§The OS HR and median OS were determined in an exploratory analysis.1
‖With 11.1 months of additional follow-up from interim analysis 2 and 19.2 months from primary analysis.4
¶The HR reported is determined using a pre-planned multivariate analysis.4
1L, first-line; AAP, abiraterone acetate (AA) + prednisone (P); ARi, androgen receptor (AR) inhibitor; BICR, blinded independent central review; BM, biomarker; BPI-SF, Brief Pain Inventory-Short Form; BRCAm, BRCA gene-mutated; CI, confidence interval; ctDNA, circulating tumor DNA; DAT, dual action tablet; ECOG PS, Eastern Cooperative Oncology Group Performance Status; FACT-P, Functional Assessment Cancer Therapy-Prostate; HR, hazard ratio; HRR, homologous recombination repair; mCRPC, metastatic castration-resistant prostate cancer; mCSPC, metastatic castration-sensitive prostate cancer; NE, not estimable; nmCRPC, non-metastatic castration-resistant prostate cancer; PARPi, poly-ADP ribose polymerase inhibitor; PI, prescribing information; PSA, prostate-specific antigen; rPFS, radiographic progression-free survival.
References:
- AKEEGA® [Prescribing Information]. Horsham, PA: Janssen Biotech, Inc.
- Chi KN, Sandhu S, Smith MR, et al. Niraparib plus abiraterone acetate with prednisone in patients with metastatic castration-resistant prostate cancer and homologous recombination repair gene alterations: second interim analysis of the randomized phase III MAGNITUDE trial. Ann Oncol. 2023;34(9):772-782. doi:10.1016/j.annonc.2023.06.009
- Efstathiou E, Smith MR, Sandhu S, et al. Niraparib with abiraterone acetate and prednisone in patients with metastatic castration-resistant prostate cancer and homologous recombination repair gene alterations: second interim analysis of MAGNITUDE. Presented at: the American Society of Clinical Oncology Genitourinary (ASCO-GU) Cancers Symposium; February 16-18, 2023; San Francisco, CA.
- Chi KN, Castro E, Attard G, et al. Niraparib (NIRA) with abiraterone acetate plus prednisone (AAP) as first-line (1L) therapy in patients (pts) with metastatic castration-resistant prostate cancer (mCRPC) and homologous recombination repair (HRR) gene alterations: Three-year update and final analysis of MAGNITUDE. Presented at the 2023 ESMO Congress; October 20-24, 2023; Madrid, Spain.
- Chi KN, Castro E, Attard G, et al. Niraparib and abiraterone acetate plus prednisone in metastatic castration-resistant prostate cancer: final overall survival analysis for the phase 3 MAGNITUDE trial. Eur Urol Oncol. Published online May 5, 2025. doi:10.1016/j.euo.2025.04.012
- Chi KN, Rathkopf D, Smith MR, et al. Niraparib and abiraterone acetate for metastatic castration-resistant prostate cancer. J Clin Oncol. 2023;41(18):3339-3351. doi:10.1200/JCO.22.01649
