Benefit of 2-in-1
AKEEGA® is the only FDA-approved medication that combines PARPi/NHT into a single dual action tablet1
Simplified dosing in a once-daily* dual action tablet

2 THERAPIES

1 CO-PAY

1 PRIOR AUTHORIZATION
The recommended AKEEGA® dose is 200 mg niraparib/1,000 mg abiraterone acetate


*AKEEGA® is indicated with 10 mg prednisone daily.1
Flexible dosing
AKEEGA®† offers your patients 2 strengths for dosing flexibility and adjustments as needed1‡


AKEEGA® recommended dose
200 mg/1,000 mg (niraparib/abiraterone acetate) once daily with 10 mg of prednisone daily


AKEEGA® reduced-dose options for specific ARs
2 lower-strength (50 mg/500 mg) niraparib/
abiraterone acetate once daily with 10 mg of prednisone daily
-OR-


1 regular-strength (100 mg/500 mg) niraparib/
abiraterone acetate once daily with 10 mg of prednisone daily
†AKEEGA® is indicated with 10 mg prednisone daily. Patients receiving AKEEGA® should also receive a GnRH analog concurrently or should have had bilateral orchiectomy.1
‡See full Prescribing Information for guidance on dosage modification for adverse reactions with AKEEGA®.
PILL COUNT vs OLAPARIB
Reduce your patients’ daily pill count with AKEEGA® compared with Lynparza® (olaparib)1,4§
Recommended daily dosage for FDA-approved PARPi combination therapies that include abiraterone acetate1,4

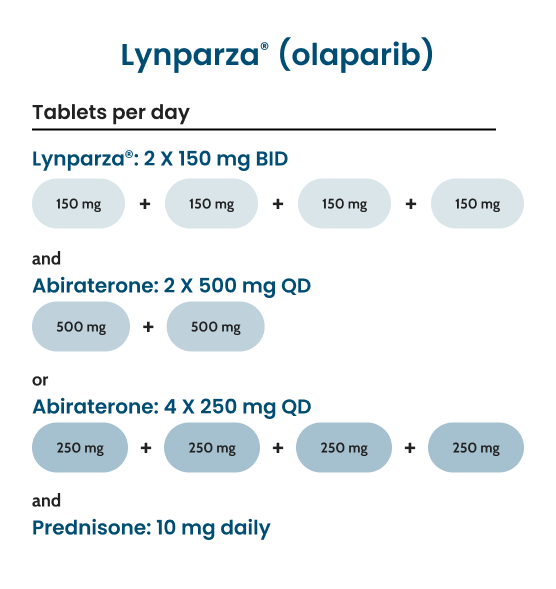
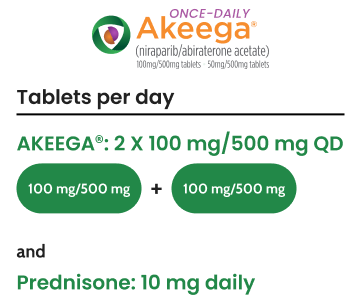

There have been no head-to-head clinical trials comparing the efficacy or safety of AKEEGA® to other PARPi treatments for prostate cancer. No direct evaluation of comparative clinical profiles can be made.
§Compared with Lynparza® + abiraterone acetate, AKEEGA® reduces patients’ daily pill count for patients with BRCAm mCRPC.4

AKEEGA® Safety & Dosing Guide
Supportive resource to help manage dosing and ARs in your patients
Administration
The recommended dose of AKEEGA® is 200 mg niraparib/1,000 mg abiraterone acetate (two 100 mg/500 mg tablets)1†

AKEEGA® tablets must be taken orally as a single dose once a day on an empty stomach

Patients should not eat food for at least 2 hours before and for at least 1 hour after taking AKEEGA®

The tablets must be swallowed whole with water. Advise patients not to crush or chew tablets
If a dose of AKEEGA® or prednisone is missed, instruct patients to take the dose as soon as possible on the same day and to return to the normal schedule the following day

Extra tablets must not be taken to make up for the missed dose
†AKEEGA® is indicated with 10 mg prednisone daily. Patients receiving AKEEGA® should also receive a GnRH analog concurrently or should have had bilateral orchiectomy.1
References:
- AKEEGA® [Prescribing Information]. Horsham, PA: Janssen Biotech, Inc.
- Schiewer MJ, Goodwin JF, Han S, et al. Dual roles of PARP-1 promote cancer growth and progression. Cancer Discov. 2012;2(12):1134-1149. doi:10.1158/2159-8290.CD-12-0120
- Teyssonneau D, Margot H, Cabart M, et al. Prostate cancer and PARP inhibitors: progress and challenges. J Hematol Oncol. 2021;14(1):51. doi:10.1186/s13045-021-01061-x
- Data on file. Janssen Biotech, Inc.
