
BRCAm in prostate cancer
BRCAm mCRPC PROGNOSIS
Testing for BRCA can inform treatment decisions1
Your patients with BRCAm mCRPC may face aggressive disease and worse prognosis2-5
~10% of patients with mCRPC harbor a BRCA gene mutation6,7
Identifying patients’ mutational status is critical to potentially changing the course of their disease in advanced prostate cancer3,8
Your patients with mCRPC may benefit from getting retested for BRCA as their tumor could mutate over time.9
Patients with BRCAm mCRPC may face shortened survival and treatment duration on novel hormonal therapies8,10
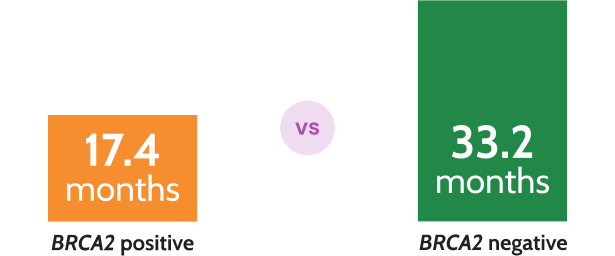
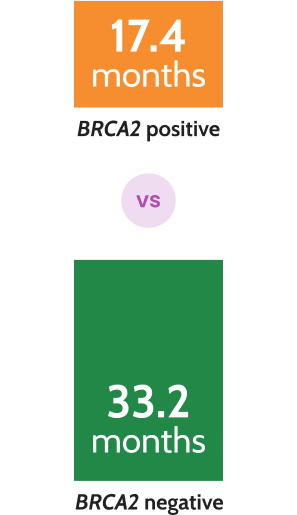
Prostate cancer survival was halved in patients with BRCA2 mutations compared to those without in mCRPC8*
N=419, P=0.027
*In a multicenter prospective cohort study of 419 patients with mCRPC, prostate cancer-specific survival was halved in those with germline BRCA2 mutations (17.4 vs 33.2 months; P=0.027).8
Testing for BRCA
Treating with AKEEGA® starts by testing for BRCA1
Is AKEEGA® appropriate for your patient?
The NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) recommend both somatic and germline testing for BRCA1/2 mutations in all patients with metastatic prostate cancer11
- The panel strongly recommends a metastatic biopsy for histologic and molecular evaluation. When unsafe or unfeasible, plasma ctDNA assay is an option, preferably collected during PSA and/or radiographic progression in order to maximize diagnostic yield
- Patients should be informed that tumor testing may uncover germline findings, and confirmatory germline testing may be recommended to inform familial risk
Using an FDA-approved diagnostic test, find out if your patient may benefit from AKEEGA®1
| Tumor tissue or ctDNA testing | Blood or saliva testing | |
|---|---|---|
| Types of tests | Either tumor tissue or ctDNA testing may detect both germline and somatic mutations but is unable to distinguish between the two3 | Both blood and saliva testing can only detect germline mutations but can also inform familial risk and cascade testing11 |
| When to test | National Comprehensive Cancer Network® (NCCN®) recommends somatic testing for all patients with metastatic prostate cancer11 | NCCN recommends germline testing at prostate cancer diagnosis for patients at high risk of certain mutations, such as BRCA1/211 |
Dual action benefits
Only AKEEGA® combines the precision of PARP inhibition with an NHT into a dual action tablet1


Guidelines
AKEEGA® is the ONLY FDA-approved dual action tablet for BRCAm mCRPC1
NCCN Guidelines® recommend niraparib and abiraterone acetate as a treatment option in certain patients11†‡:
| Prior docetaxel11 | Prior NHT11 | NCCN Treatment Recommendation11 |
|---|---|---|
| ✗ | ✗ | NCCN CATEGORY 1 |
| ✓ | ✗ | NCCN CATEGORY 2A |
| ✗ | ✓ | NCCN CATEGORY 2B |
†See the NCCN Guidelines® for detailed recommendations, including other treatment options.
‡Continue ADT to maintain castrate levels of serum testosterone (<50 ng/dL).11
§Category 1: Based upon high-level evidence (≥1 randomized phase 3 trials or high-quality, robust meta-analyses), there is uniform NCCN consensus (≥85% support of the Panel) that the intervention is appropriate.11
||Category 2A: Based upon lower-level evidence, there is uniform NCCN consensus (≥85% support of the Panel) that the intervention is appropriate.11
¶Category 2B: Based upon lower-level evidence, there is NCCN consensus (≥50%, but <85% support of the Panel) that the intervention is appropriate.11
ASCO Guidelines recommend niraparib and abiraterone acetate for the treatment of patients with BRCAm mCRPC who have been previously treated with ADT ± docetaxel14
ADT, androgen deprivation therapy; AR, androgen receptor; ASCO, American Society of Clinical Oncology; BRCAm, BRCA gene-mutated; ctDNA, circulating tumor DNA; DNA, deoxyribonucleic acid; FDA, U.S. Food and Drug Administration; mCRPC, metastatic castration-resistant prostate cancer; NCCN, National Comprehensive Cancer Network; NHT, novel hormonal therapy; PARP, poly (ADP-ribose) polymerase; PSA, prostate-specific antigen.
References:
- AKEEGA® [Prescribing Information]. Horsham, PA: Janssen Biotech, Inc.
- Teyssonneau D, Margot H, Cabart M, et al. Prostate cancer and PARP inhibitors: progress and challenges. J Hematol Oncol. 2021;14(1):51.
- Scott RJ, Mehta A, Macedo GS, et al. Genetic testing for homologous recombination repair (HRR) in metastatic castration-resistant prostate cancer (mCRPC): challenges and solutions. Oncotarget. 2021;12(16):1600-1614. doi:10.18632/oncotarget.28015
- Giri VN, Morgan TM, Morris DS, et al. Genetic testing in prostate cancer management: considerations informing primary care. CA Cancer J Clin. 2022;72(4):360-371. doi:10.3322/caac.21720
- Messina C, Cattrini C, Soldato D, et al. BRCA mutations in prostate cancer: prognostic and predictive implications. J Oncol. 2020;2020:4986365. doi: 10.1155/2020/4986365
- Shore N, Oliver L, Shui I, et al. Systematic literature review of the epidemiology of advanced prostate cancer and associated homologous recombination repair gene alterations. J Urol. 2021;205(4):977-986. doi:10.1097/JU.0000000000001570
- George DJ, Khilfeh I, Rossi C, et al. Real-world prevalence of select homologous recombination repair (HRR) alterations in patients with metastatic castration-resistant prostate cancer (mCRPC). Poster presented at: the 23rd Annual Meeting of the Society of Urologic Oncology; November 30-December 2, 2022; San Diego, CA.
- Castro E, Romero-Laorden N, Del Pozo A, et al. PROREPAIR-B: a prospective cohort study of the impact of germline DNA repair mutations on the outcomes of patients with metastatic castration-resistant prostate cancer. J Clin Oncol. 2019;37(6):490-503. doi:10.1200/JCO.18.00358
- Cimadamore A, Lopez-Beltran A, Massari F, et al. Germline and somatic mutations in prostate cancer: focus on defective DNA repair, PARP inhibitors and immunotherapy. Future Oncol. 2020;16(5):75-80. doi:10.2217/fon-2019-0745
- Annala M, Struss WJ, Warner EW, et al. Treatment outcomes and tumor loss of heterozygosity in germline DNA repair-deficient prostate cancer. Eur Urol. 2017;72(1):34-42. doi:10.1016/j.eururo.2017.02.023
- Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Prostate Cancer V.2.2025 © National Comprehensive Cancer Network, Inc. 2025. All rights reserved. Accessed June 18, 2025. To view the most recent and complete version of the guideline, go online to NCCN.org. NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way.
- Schiewer MJ, Goodwin JF, Han S, et al. Dual roles of PARP-1 promote cancer growth and progression. Cancer Disc. 2012;2(12):1134-1149.
- Asim M, Tarish F, Zecchini HI, et al. Synthetic lethality between androgen receptor signaling and the PARP pathway in prostate cancer. Nat Commun. 2017;8(1):374.
- Garje R, Riaz IB, Naqvi SAA, et al. Systemic therapy in patients with metastatic castration-resistant prostate cancer: ASCO guideline update. Supplementary appendix. J Clin Oncol. Published online May 2, 2025. doi:10.1200/JCO-25-00007